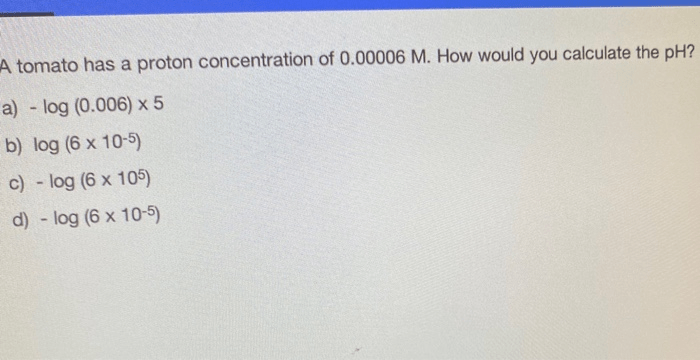
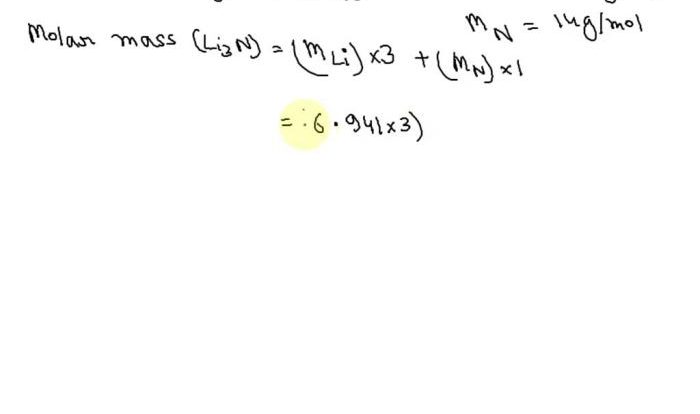A tomato has a proton concentration of 0.00006 m – With a proton concentration of 0.00006 M, tomatoes offer a fascinating glimpse into the world of pH and its implications. This comprehensive guide delves into the concept of proton concentration, its measurement, and its significance in biological and industrial contexts, revealing the hidden chemistry behind this ubiquitous fruit.
Delving into the intricacies of proton concentration, we explore its role in defining acidic and basic solutions, unraveling the mysteries of pH measurement, and uncovering its diverse applications. From maintaining cellular homeostasis to optimizing industrial processes, the importance of proton concentration cannot be overstated.
Proton Concentration: A Tomato Has A Proton Concentration Of 0.00006 M
Proton concentration, measured in moles per liter (M), refers to the amount of hydrogen ions (H+) present in a solution. It plays a crucial role in determining the acidity or basicity of a substance.
Examples of Substances with Different Proton Concentrations
- Pure water: 1 x 10^-7 M
- Lemon juice: 1 x 10^-2 M
- Battery acid: 1 M
pH Measurement
Relationship between Proton Concentration and pH
pH is a logarithmic measure of proton concentration. The pH scale ranges from 0 to 14, with lower pH values indicating higher proton concentrations and higher acidity.
Measuring pH using a pH Meter
A pH meter is an instrument that measures the pH of a solution by detecting the electrical potential between a glass electrode and a reference electrode. The electrical potential is directly proportional to the proton concentration.
Acidic vs. Basic Solutions
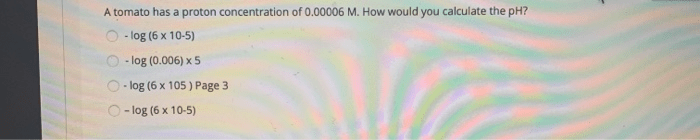
Definition Based on Proton Concentration
Acidic solutions have a higher proton concentration (lower pH) than pure water, while basic solutions have a lower proton concentration (higher pH) than pure water.
Examples of Acidic and Basic Substances, A tomato has a proton concentration of 0.00006 m
- Acidic: Hydrochloric acid (HCl), sulfuric acid (H2SO4)
- Basic: Sodium hydroxide (NaOH), potassium hydroxide (KOH)
Applications of Proton Concentration

Importance in Biological Systems
Proton concentration is vital for maintaining cellular homeostasis and regulating enzyme activity in biological systems.
Industrial Processes
Proton concentration is used in various industrial processes, including:
- Electroplating
- Battery production
- Water purification
Safety Considerations
Potential Hazards
Handling solutions with high proton concentrations can be hazardous, causing:
- Skin burns
- Eye damage
- Respiratory irritation
Safety Precautions
To minimize risks, follow these precautions:
- Wear protective clothing and gloves.
- Handle solutions in well-ventilated areas.
- Dispose of solutions properly.
FAQ Guide
What is the significance of a tomato’s proton concentration?
Tomatoes have a proton concentration of 0.00006 M, which indicates a slightly acidic nature. This acidity contributes to their characteristic flavor and plays a role in preserving their nutritional value.
How does proton concentration affect biological systems?
Proton concentration is crucial for maintaining cellular homeostasis, enzyme activity, and overall metabolic processes. Deviations from optimal proton concentrations can disrupt cellular functions and lead to various health conditions.
What are some industrial applications of proton concentration?
Proton concentration is utilized in various industrial processes, including food preservation, water treatment, and chemical manufacturing. By controlling pH levels, industries can optimize product quality, efficiency, and safety.