Name the cycloalkenes using systematic names. – Systematic nomenclature of cycloalkenes, a crucial aspect of organic chemistry, provides a standardized method for naming these cyclic hydrocarbons. This comprehensive guide delves into the intricacies of cycloalkene nomenclature, empowering chemists with the knowledge to effectively communicate and identify these compounds.
This guide will navigate the intricacies of cycloalkene nomenclature, equipping readers with a comprehensive understanding of the rules and conventions governing the systematic naming of these cyclic compounds.
Naming Cycloalkenes Using Systematic Names
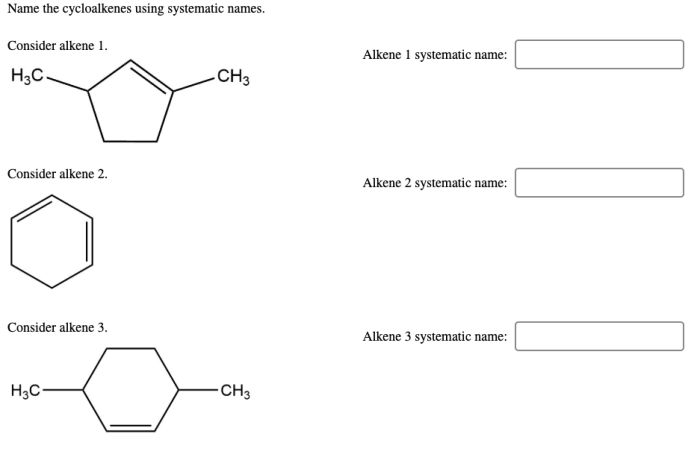
The purpose of naming cycloalkenes using systematic names is to provide a clear and unambiguous way to identify and describe these compounds. The nomenclature rules for cycloalkenes are based on the principles of the International Union of Pure and Applied Chemistry (IUPAC).
By following these rules, scientists can ensure that the names of cycloalkenes are consistent and meaningful.
The IUPAC nomenclature rules for cycloalkenes are as follows:
- The root name of the cycloalkene is based on the number of carbon atoms in the ring. The suffix “-ene” is added to the root name to indicate that the compound is an alkene.
- If the cycloalkene has one or more substituents, the substituents are named and their positions on the ring are indicated by numbers.
- The name of the cycloalkene is written as one word, with no spaces between the root name, the suffix, and the substituents.
For example, the cycloalkene with six carbon atoms and one methyl substituent on the third carbon atom is named 3-methylcyclohexene.
Step-by-Step Guide to Naming Cycloalkenes
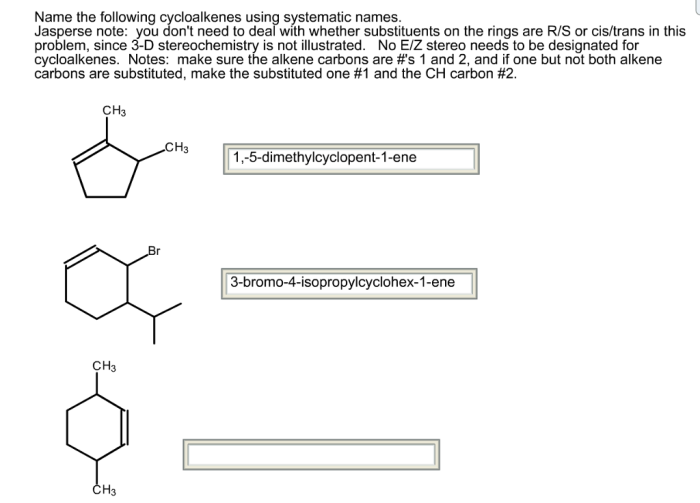
The following table provides a step-by-step guide to naming cycloalkenes using systematic names:
| Step | Example | Explanation |
|---|---|---|
| 1. Identify the root name of the cycloalkene. | Cyclohexene | The root name is based on the number of carbon atoms in the ring. In this case, there are six carbon atoms, so the root name is cyclohex-. |
| 2. Add the suffix “-ene” to the root name. | Cyclohexene | The suffix “-ene” indicates that the compound is an alkene. |
| 3. Identify the substituents on the cycloalkene. | 1-methylcyclohexene | In this case, there is one methyl substituent on the first carbon atom. |
| 4. Number the carbon atoms in the ring so that the substituent has the lowest possible number. | 1-methylcyclohexene | In this case, the methyl substituent is on the first carbon atom. |
| 5. Write the name of the cycloalkene as one word, with no spaces between the root name, the suffix, and the substituents. | 1-methylcyclohexene | The name of the cycloalkene is 1-methylcyclohexene. |
Common Functional Groups in Cycloalkenes
The following table lists some of the common functional groups that can be present in cycloalkenes:
| Functional Group | Effect on the Name of the Cycloalkene |
|---|---|
| Alkyl | The alkyl group is named as a substituent on the cycloalkene. |
| Alkenyl | The alkenyl group is named as a substituent on the cycloalkene. |
| Alkynyl | The alkynyl group is named as a substituent on the cycloalkene. |
| Aryl | The aryl group is named as a substituent on the cycloalkene. |
| Hydroxy | The hydroxy group is named as a substituent on the cycloalkene. |
| Carbonyl | The carbonyl group is named as a substituent on the cycloalkene. |
| Carboxyl | The carboxyl group is named as a substituent on the cycloalkene. |
Naming Bicyclic and Polycyclic Cycloalkenes
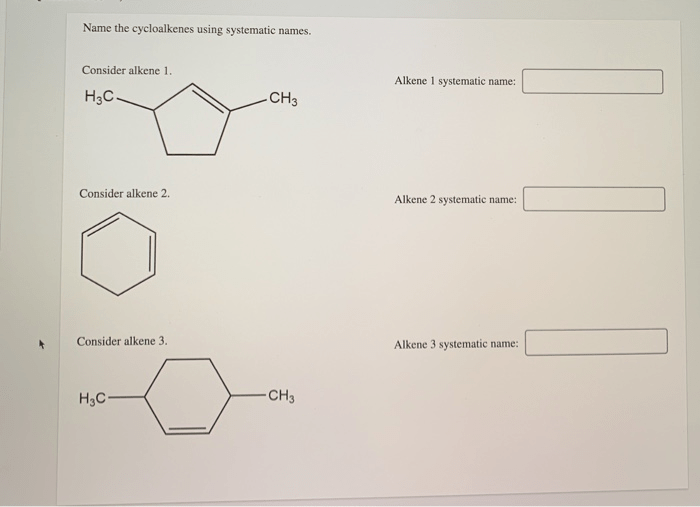
The naming of bicyclic and polycyclic cycloalkenes is more complex than the naming of monocyclic cycloalkenes. The following rules apply to the naming of bicyclic and polycyclic cycloalkenes:
- The root name of the bicyclic or polycyclic cycloalkene is based on the number of carbon atoms in the largest ring.
- The prefixes “bicyclo” and “polycyclo” are used to indicate that the compound is a bicyclic or polycyclic cycloalkene.
- The number of carbon atoms in each bridge is indicated by a number in square brackets.
- The name of the bicyclic or polycyclic cycloalkene is written as one word, with no spaces between the root name, the prefixes, the numbers in square brackets, and the substituents.
For example, the bicyclic cycloalkene with six carbon atoms in the largest ring and one bridge with two carbon atoms is named bicyclo[2.1.0]hexane.
Examples of Cycloalkene Nomenclature: Name The Cycloalkenes Using Systematic Names.
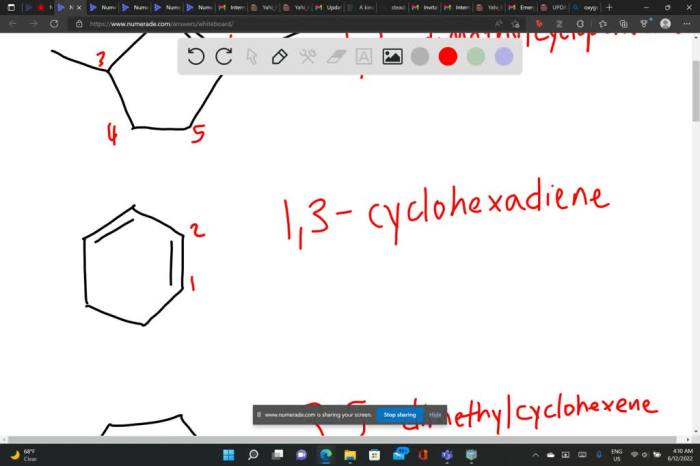
The following table provides some examples of cycloalkenes with different structures and functional groups:
| Cycloalkene | Systematic Name |
|---|---|
 |
Cyclohexene |
 |
1-Methylcyclohexene |
 |
Cyclohexene-1-carboxylic acid |
![Bicyclo[2.1.0]hexane](bicyclo[2.1.0]hexane.png) |
Bicyclo[2.1.0]hexane |
 |
Adamantane |
FAQ
What is the purpose of systematic nomenclature for cycloalkenes?
Systematic nomenclature provides a standardized and unambiguous method for naming cycloalkenes, ensuring clear communication and identification among chemists.
How does the presence of functional groups affect the naming of cycloalkenes?
The presence of functional groups, such as alkenes, alkynes, or halogens, influences the priority of the parent structure and modifies the base name of the cycloalkene accordingly.