Embark on a scientific adventure as we delve into the molar mass of lithium nitride, a fascinating compound with a diverse range of applications. From its role in fertilizers to its use in ceramics and glass manufacturing, this multifaceted material holds secrets that we are eager to uncover.
As we journey through the composition of lithium nitride, we will unravel the elements that make up its structure and calculate its molar mass. Along the way, we will discover the significance of molar mass in chemistry and explore the practical implications of this compound in various industries.
Introduction to Molar Mass
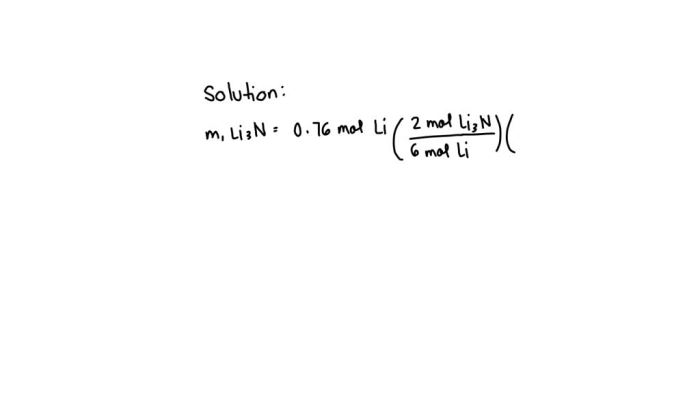
In chemistry, molar mass is a fundamental concept that plays a crucial role in understanding the composition and properties of substances. It represents the mass of one mole of a substance and is a crucial parameter for various calculations and conversions.
Formula for Calculating Molar Mass
The molar mass of a substance is calculated by adding the atomic masses of all the atoms present in its molecular formula. The atomic mass of an element is the weighted average mass of all its isotopes, taking into account their relative abundances.
Molar mass = Sum of atomic masses of all atoms in the molecular formula
Composition of Lithium Nitride
Lithium nitride is a compound composed of two elements: lithium and nitrogen. Its chemical formula is Li 3N. To calculate the molar mass of lithium nitride, we first need to determine the molar mass of each of its constituent elements.
Molar Mass of Lithium
- Atomic number of lithium: 3
- Atomic mass of lithium: 6.941 amu
- Molar mass of lithium: 6.941 g/mol
Molar Mass of Nitrogen
- Atomic number of nitrogen: 7
- Atomic mass of nitrogen: 14.007 amu
- Molar mass of nitrogen: 14.007 g/mol
Determining Molar Mass of Lithium Nitride
Calculating the molar mass of lithium nitride involves determining the sum of the atomic masses of its constituent elements.
Calculating Molar Mass
The molar mass of lithium nitride (Li3N) can be calculated using the formula:
Molar mass = (Atomic mass of lithium) × 3 + (Atomic mass of nitrogen)
The atomic mass of lithium (Li) is 6.941 amu, and the atomic mass of nitrogen (N) is 14.007 amu.
Example Calculation, Molar mass of lithium nitride
Substituting the atomic masses into the formula, we get:
Molar mass of Li3N = (6.941 amu × 3) + (14.007 amu) = 34.855 amu
Therefore, the molar mass of lithium nitride is 34.855 amu.
The molar mass of lithium nitride is a crucial aspect to consider in various chemical reactions. If you’re looking for a comprehensive resource on advanced cardiovascular life support, I highly recommend checking out the acls test answers version a . This invaluable guide provides detailed explanations and practice questions to enhance your understanding of ACLS protocols.
Returning to the topic of lithium nitride, its molar mass plays a significant role in determining its reactivity and other properties.
Applications of Lithium Nitride
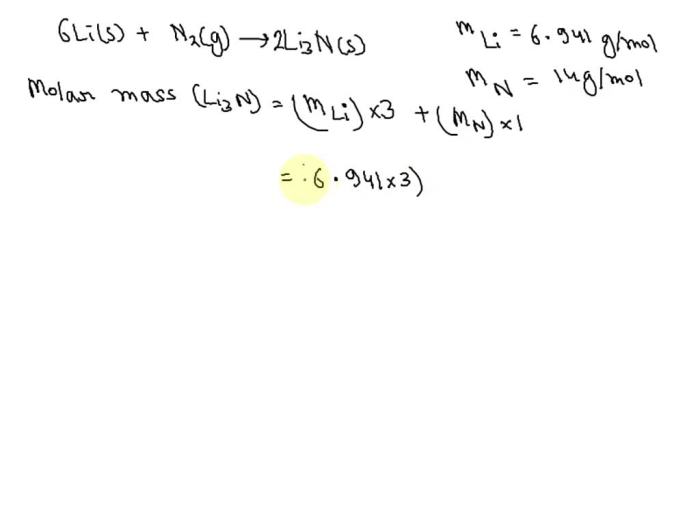
Lithium nitride is a versatile compound with a wide range of industrial applications. It is primarily used as a source of nitrogen in fertilizers, as well as in the manufacturing of ceramics and glass.
Role in Fertilizers
Lithium nitride is a valuable nitrogen source for fertilizers. Nitrogen is essential for plant growth, and lithium nitride provides a convenient and effective way to deliver it to crops. When applied to soil, lithium nitride reacts with water to release ammonia, which is then absorbed by plants.
Applications in Ceramics and Glass Manufacturing
Lithium nitride is also used in the production of ceramics and glass. In ceramics, it acts as a flux, lowering the melting point of the ceramic mixture and promoting the formation of a glassy phase. In glass manufacturing, lithium nitride is added to the glass batch to improve its thermal stability and resistance to chemical attack.
Clarifying Questions
What is the molar mass of lithium nitride?
The molar mass of lithium nitride is approximately 34.85 g/mol.
What are the elements that make up lithium nitride?
Lithium nitride is composed of lithium and nitrogen.
What is the chemical formula of lithium nitride?
The chemical formula of lithium nitride is Li3N.
What are the industrial uses of lithium nitride?
Lithium nitride is used in the production of fertilizers, ceramics, and glass.
